1. Gluten is a type of protein found in wheat, rye and barley. Gluten itself is made up of several peptides including a class of proteins called gliadins.
2. Genetics also play a role in coeliac disease, specifically the HLA-DQ2 and HLA-DQ8 alleles. These alleles code for major histocompatibility complex II proteins which are expressed by antigen presenting cells in the small intestine e.g. dendritic cells.
3. Once gliadin reaches the small intestine it is taken up by enterocytes and transported into the lamina propria of the small bowel. Several theories exist to explain how this happens, two of which include:
4. Patients with coeliac disease have high levels of tissue transglutaminase (TTG), an enzyme which deamidates gliadin into glutamic acid, or causes cross-linking of gliadin.
5. The deamidation or cross-linking of gliadin increases the likelihood of these gluten peptides being bound by HLA-DQ2 or HLA-DQ8 which will be expressed by antigen presenting cells like dendritic cells.
6. Antigen presenting cells will present these antigens to CD4+ T cells, specifically T-helper 1 cells. In response to this, activated CD4+ T cells will release inflammatory cytokines such as interferon-gamma and IL-1, which ultimately results in apoptosis of enterocytes.[ii]
TL;DR Gluten has proteins that can cause inflammation and destruction of enterocytes in the small bowel, which results in malabsorption and the signs and symptoms seen in coeliac disease.
Pathophysiology of Coeliac Disease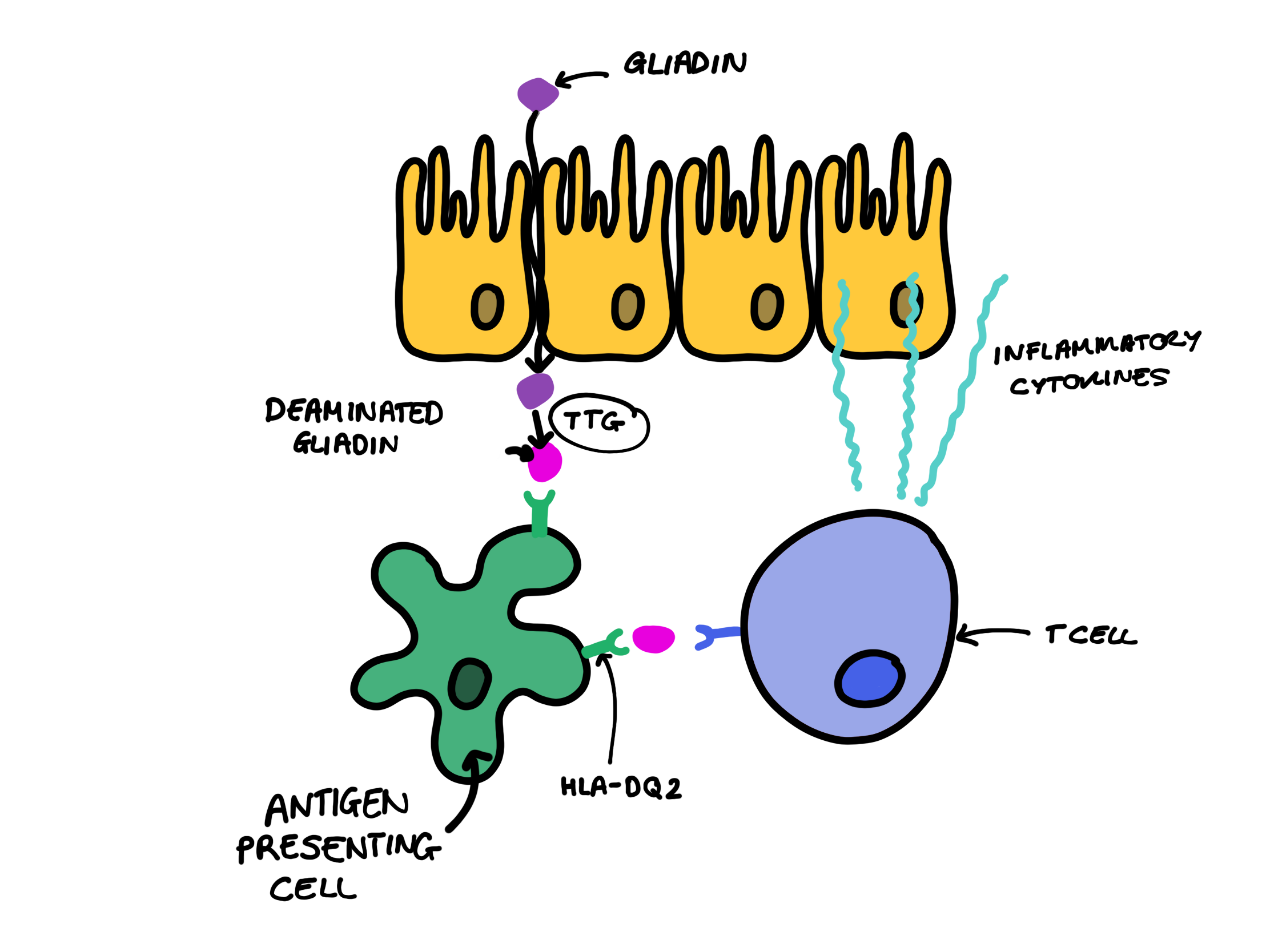
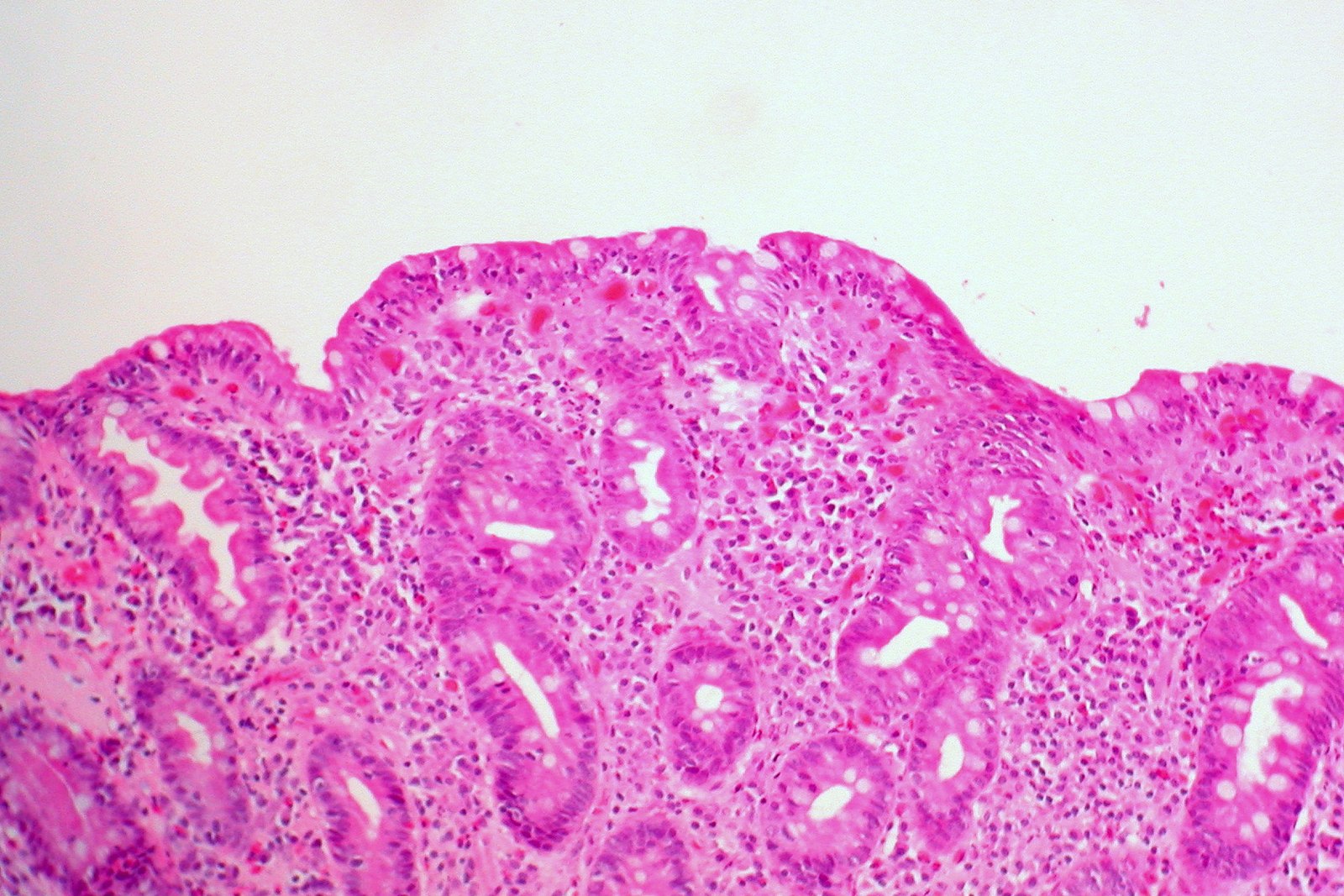
Section of the Small Bowel showing loss of normal villous architecture
Asymptomatic
Bloods
Some patients may have IgA deficiencies (characterised by a low total IgA). TTG and EMA are both IgA antibodies, so they might be negative even though someone has coeliac disease. In this case, the following tests can be done:
Genetic Testing
HLA-DQ2 or HLA-DQ8 genetic testing can also be performed, the former being positive in close to 95% of patients with coeliac disease. These are not typically used in non-specialist settings.
Endoscopy and Biopsy
A small bowel biopsy is the gold-standard diagnostic method. Classic histological features will confirm the diagnosis. The British Society of Gastroenterologists recommend that at least 4 biopsies be obtained, with at least one being from the duodenal bulb.[vi] Patients must be on a gluten containing diet during the time of the biopsy.
A gluten free diet is crucial in patients with coeliac disease since gluten is so damaging for the small bowel. Thus, products such as bread, flour, pasta, crackers, pastries, cereals etc will need to be eliminated from the diet. Anti-tTG and anti-EMA titres correspond to disease severity and can therefore be used to monitor response to gluten eradication.
If a patient has severe dermatitis herpetiformis, they may require dermatology referral.
[i] Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. [internet]. 2012. [cited 26th August 2019]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3384703/
[ii] Sabatino AD, Corazza GR. Coeliac disease. [internet]. 2009. [cited 26th August 2019]. Available from: https://www.sciencedirect.com/science/article/pii/S0140673609602543
[iii] Young B, O’Dowd G and Woodford P. Wheater’s Functional Histology. 6e. Elsevier Ltd. 2014.
[iv] Pathology Outlines. Celiac sprue. [internet]. 2019. [cited 26th August 2019]. Available from: http://www.pathologyoutlines.com/topic/smallbowelceliacsprue.html
[v] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC130112/
https://cks.nice.org.uk/topics/coeliac-disease/diagnosis/assessment/
[vii] Medscape. Risk of Morbidity in Contemporary Celiac disease. [internet]. 2010. [cited 30th August 2019]. Available from: https://www.medscape.com/viewarticle/734493_10