Parkinson’s disease is a neurodegenerative disease characterised mainly by motor symptoms, alongside other features.
Pathophysiology
- The basal ganglia is a group of various structures in the brain that work together to help regulate motor function. The basal ganglia also interacts with the amygdala, meaning the structures that make up the basal ganglia also help regulate decision making and behaviour.
- In simple terms, every time the motor cortex initiates a movement, the basal ganglia will work to inhibit any unwanted movements or help to initiate wanted movements. Diseases of the basal ganglia will lead to abnormal control of movements, unwanted movements, and changes in posture.
- For fine-tuning of motor control, there is an area called the substantia nigra which communicates with the basal ganglia via dopaminergic neurones which release the neurotransmitter dopamine.
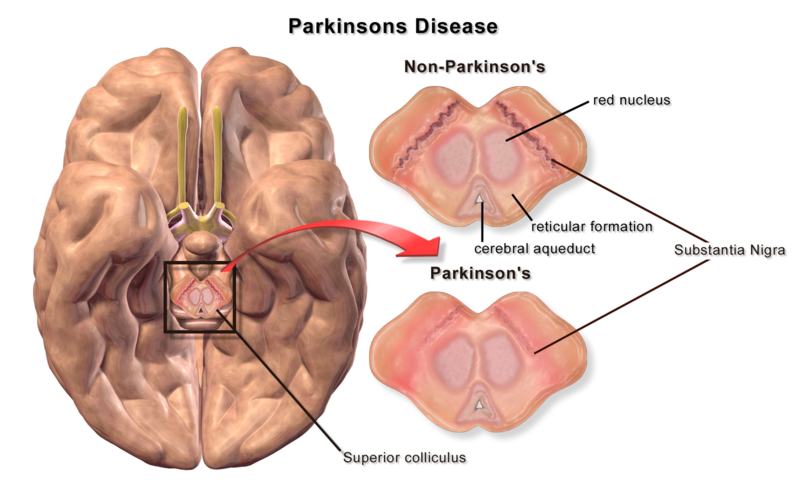
- In Parkinson’s disease, there is a loss of the dopaminergic excitatory cells of the pars compacta of the substantia nigra. Dopamine normally excites the neurones of part of the basal ganglia to help initiate movement – thus a loss of dopamine will make it more difficult to initiate movements.
- The dopamine cells which are lost are actually pigmented. When observing the midbrain of a patient (in a cadaveric specimen - don't go doing that on ward patients please) who has had Parkinson’s disease, the depigmentation of the substantia nigra is clearly visible.
BruceBlaus. When using this image in external sources it can be cited as:Blausen.com staff (2014).
Associations
- A protein called alpha-synuclein can build up and deposit in the cells of the substantia nigra – these build ups are known as Lewy bodies.
- Lewy bodies can also deposit in other cells of the brain, leading to neuronal cell death and subsequent Lewy body dementia.
- As a result, many patients with Parkinson’s disease will go on to develop Lewy body dementia.
- If symptoms of dementia present first, followed by symptoms of Parkinson’s disease, this patient is said to have Parkinson’s disease dementia.
Clinical Features
- Parkinson's Disease Triad
- Bradykinesia (Slow movements)
- Resting tremor: Usually a ‘pill-rolling’ quality whereby the thumb repetitively moves over the fingers. This is usually unilateral to start with.
- Rigidity: described as a ‘lead-pipe’ rigidity, meaning if you try and passively move the patient’s arm for example, no matter the speed you are moving the arm, it will be extremely rigid (as if trying to move a lead pipe).
- Loss of arm swing when walking
- Shuffling gait
- Difficulty changing direction e.g. may be slower moving around doors/furniture
- Cog-wheel rigidity: If you supinate and pronate the wrist rapidly, the wrist will ‘catch’ every so often, like a cog-wheel. This is due to the fact that patient has rigidity, as well as a resting tremor – the two combined lead to this cog-wheeling effect.
- Postural instability: Patients find it difficult to keep their balance and may be at increased risks of falls at this point.
- Stooped posture
- Dysphasia
- Flat facial expressions
- Micrographia (small handwriting)
- Dysphagia
- Hyposmia/Anosmia
Investigations
Parkinson’s disease is usually diagnosed clinically, and NICE recommend using the UK Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria for diagnosis.
In situations where there is diagnostic uncertainty, imaging may be used.
- Dopamine Transported scan (DaT) Scan: The DaT scan measures the amount of dopamine containing neurones. There will be a smaller signal coming from the tail of the basal ganglia (the putamen) in patients with Parkinson’s.
- 123I-FP-CIT single photon emission computed tomography (SPECT): Can be used when there is diagnostic uncertainty between essential tremor and Parkinsonian tremor
Differential Diagnosis
- Essential tremor: Typically, bilateral intention tremor with a positive family history. Tends to affect the hands, head and voice and also improves with alcohol.
- Drug-induced Parkinsonism: Particularly if patient has been on anti-psychotic drugs which work by inhibiting dopamine
- Wilson’s disease: Genetic disorder resulting in copper accumulation which can be associated with tremor.
Management
The main objective is to increase levels of dopamine in the brain. This is done using levodopa, a precursor to dopamine which is metabolised into dopamine in the brain and acts on dopamine receptors.
Sometimes, levodopa is prescribed with other drugs to improve the side effect profile or bioavailability of the drug. For example:
Decarboxylase Inhibitors
- Levodopa is converted into dopamine prior to crossing the blood-brain barrier i.e., in the peripheries.
- This leads to symptoms like vomiting and dizziness.
- Thus, decarboxylase inhibitors can be prescribed to prevent the peripheral conversion of levodopa into dopamine.
- Carbidopa is an example and is typically given as a combined drug with levodopa e.g. Co-careldopa = Carbidopa + levodopa
COMT Inhibitors
- In addition to this, levodopa is methylated by an enzyme called catechol-O-methyltransferase (COMT).
- This methylation reduces the drug’s bioavailability so COMT inhibitors can be used to block this from happening.
- Examples of COMT inhibitors include entacapone or tolcapone.
MAO Inhibitors
- Monoamine oxidase (MAO) breaks down dopamine when it crosses the synapse, thus reducing the amount of dopamine available in the brain.
- MAO-B inhibitors can be given to prevent this breakdown.
- Examples include selegiline or rasagiline.
Dopamine Agonists
- An alternative to levodopa is a dopamine agonist.
- However, these are not as effective as levodopa but can be better for younger patients to avoid long-term side effects of levodopa.
- There are two types of dopamine agonists – ergot derived, and non-ergot derived drugs. It is preferable to use the latter first since ergot derived drugs are thought to cause very problematic side effects such as fibrosis of heart valves.
- Examples of non-ergot derived drugs include ropinirole or rotigotine.
- Ergot derived drugs include bromocriptine and cabergoline.
Deep Brain Stimulation
Deep brain stimulation is a neurosurgical technique used to stimulate specific nuclei in the brain – for example, targeting the nuclei of the basal ganglia. It has been used to treat Parkinson’s which has been unresponsive to drug treatment.
Adding Treatments
- First-line management is usually with levodopa for management of motor symptoms
- If patients aren't too impacted from the motor symptoms, dopamine agonists and MAO-B inhibitors can be considered as alternative first-lines
- In instances where patients start to develop long-term problems from levodopa (mentioned below), a combination of COM-T inhibitors, MAO-B inhibitors or dopamine agonists can be used alongside the levodopa.
Other Management
- Drug treatment alone is usually insufficient – a holistic approach to treatment should be taken. Patients may benefit from a specialist nurse, occupational therapy, speech and language input, physiotherapy and dietetics advice.
- Additionally, offering patients symptomatic relief for the non-motor symptoms such as anti-cholinergics for treating excessive drooling or anti-depressants for low mood.
- Modafanil can be used for daytime sleepiness
- Midodrine can be used for postural hypotensions which can be seen in PD
Long-Term Problems with Levodopa
Patients being treated for Parkinson’s disease tend to start off with a ‘honeymoon period’ whereby they respond well to treatment. After some time, it becomes increasingly difficult to treat the disease and more and more dopamine is required to control symptoms.
- Wearing off: The amount of time levodopa will work for starts to reduce i.e. the drug is ‘wearing off’ quicker.
- On/off phenomenon: This is when patients have sudden and unpredictable fluctuations between immobility and mobility due to being either ‘on’ or ‘off.’ When ‘on’, patients respond well to levodopa but when ‘off’, there is little response to the levodopa so symptoms arise. Apomorphine infusions can be given to help with this – since this is given as an infusion, it constantly stimulates dopamine receptors which may be helpful against motor fluctuations.
- Dyskinesias: These can start to develop as a result of high amounts of dopamine. Dyskinesias are abnormal, uncontrollable and involuntary movements. Amantadine has been used to help with this.
References
https://www.nice.org.uk/guidance/ng71/chapter/Recommendations#diagnosing-parkinsons-disease
Parkinson’s UK. Apomorphine. [internet]. 2017. [cited 1st July 2019]. Available from: https://www.parkinsons.org.uk/sites/default/files/2018-03/FS26%20Apomorphine%20WEB.pdf
Medscape. Parkinson Disease Clinical Presentation. [internet]. 2019. [cited 1st July 2019]. Available from: https://emedicine.medscape.com/article/1831191-clinical
ParkinsonsDisease.net. What are COMT inhibitors? [internet]. 2019. [cited 1st July 2019]. Available from: https://parkinsonsdisease.net/medications/comt-inhibitors/